For decades, a diagnosis of glioblastoma – an aggressive, hard-to-treat cancer in the brain – has been a death sentence for patients.
Only 3% to 5% of people who are diagnosed with this type of brain tumor will be alive three years later. On average, patients live about 14 months after diagnosis.
Now, an experimental therapy that reprograms a person’s own immune cells to attack these tumors is showing some exciting promise.
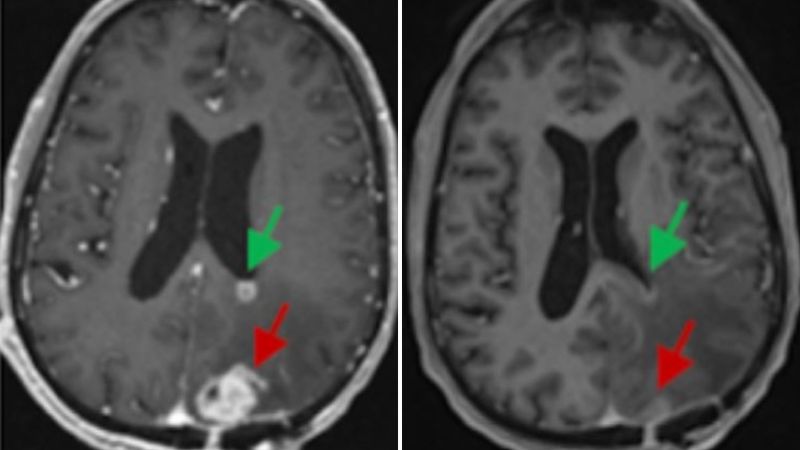
Three studies published within the past week have reported dramatic results with a therapy called CAR-T delivered directly to the brain. In some cases, tumors have seemingly melted away on brain scans by the next day.
“That was shocking to me,” said Dr. Otis Brawley, a professor of oncology at Johns Hopkins University and former chief medical officer of the American Cancer Society, who was not involved in the research. “That’s fast. I mean, whoa!”
In most cases, however, the tumors have returned, and none of the studies – from the City of Hope Cancer Center in Duarte, California; the University of Pennsylvania; and Massachusetts General Hospital – has demonstrated a survival benefit for patients. But researchers think that with some tweaks, they’ll soon be able to accomplish that.
“They clearly made the tumors shrink, so it’s doing something,” Brawley said, “Now, the hard part starts.
“We have a drug that has some activity. We have to figure out how we can maximize that activity,” he said.
‘It has given me hope’
Tom Fraser, 72, of Rochester, New York, signed up for the pilot study of CAR-T at Mass General Brigham last summer to treat a glioblastoma tumor in his brain that had continued to grow despite chemotherapy and radiation. The results of the study were published Wednesday in the New England Journal of Medicine.
Doctors first harvested immune fighters called T-cells from his blood and then genetically modified them in a lab so they’d recognize and bind to specific proteins on the surface of the brain tumor cells. The Mass General researchers also took a second step: adding another modification to the CAR-T cells to help his body outsmart the cancer.
Tumors are devious because they can co-opt the body’s immune function to hide from detection, in this case making use of a different kind of T-cell, called a suppressor T-cell, that turns down immune responses. The CAR-T cells that Fraser got had a modification that reprogrammed the suppressor T-cells protecting the tumor to become killer T-cells that would fight it instead.
After a single 10-milliliter infusion of about 10 million CAR-T cells, Fraser’s tumor began to shrink. On an MRI scan the next day, it was nearly 20% smaller, and within weeks, it was barely detectable. He’s seen no progression of his cancer for about six months now, according to his doctors. He’s just had his third brain surgery.
“These kinds of responses don’t really happen with any other kinds of therapy for glioblastoma,” said Dr. Marcela Maus, lead author of the study.
Fraser said in emailed responses to questions that he had the kind of side effects he was warned to expect with the CAR-T, which included a fever and extreme fatigue, “but as time went on, I was able to resume life including exercise classes, spending time with family and friends and have been able to travel.”
“It has given me hope personally and also given me hope that this will lead to a cure!” Fraser wrote.
Two other patients had their tumors shrink after a single treatment, but their cancers returned one month and two months after their infusions.
Still, the researchers say they are encouraged by what they saw.
“Even though two of our patients progressed before six months, we think that we can do various maneuvers to try to increase that durability,” said Maus, director of the cellular immunotherapy program at Mass General Cancer Center in Boston.
She says they are considering giving chemotherapy before CAR-T, for example, to try to improve the protocol.
Maus stresses that this was just a pilot study designed to find the right dose to give patients and to make sure it was safe to give to others. Getting these kinds of responses in their first three patients was unexpected, she said.
“We were so surprised by how much happened in the first patient and so quickly, and then to see it again two more times,” Maus said. “To me it just says, like, this has legs.”
Versions of this therapy, which is officially called CAR-Tv3-TEAM-E, have been used for blood cancers like leukemia and lymphoma since 2017.
Now, researchers are learning how to apply the same technology to solid tumors, and the results look promising – but not permanent. This is not a cure.
“We are still at the early stages,” said Dr. Christine Brown, deputy director of the T-Cell Therapeutics Research Laboratory at the City of Hope Cancer Center. “There are patients that are showing very favorable responses, but we still have too many patients that are progressing through therapy.
“This is not yet a magic bullet, but we’re hoping that we’re getting closer to something that’s meaningful for patients,” she said.
Tumors that ‘melted away’
Brown and her team recently published results from the largest study yet of the use of CAR-T in advanced glioma brain tumors, which included 65 patients. All had already been through standard treatments like chemotherapy, radiation and surgery. Three-quarters of the participants had had their brain tumors come back at least twice.
After the CAR-T therapy, two of the participants had complete responses, meaning their tumors disappeared.
One person, whose case was initially reported in 2016, had been told that he had weeks to live. But after getting six infusions of CAR-T therapy directly into the spinal fluid that bathes the brain, his tumors “melted away,” Brown said. “That really set this the foundation, or the stage, for us to try to understand why he responded so strongly and the therapy worked so well,” she added.
The CAR-T therapy being tested at City of Hope is slightly different than the one used at Mass General to help the T-cells find and kill the brain tumor. Their CAR-T targets a protein made by tumors called interleukin-13 receptor alpha 2.
The other patient who saw his tumor completely disappear had been through three surgeries to remove a tumor on the right side of his brain before doctors told him they couldn’t operate anymore. After CAR-T at City of Hope in 2018, his tumor has gone away, and he hasn’t had a recurrence of his cancer in 5½ years.
But Brown stresses that these two cases were outliers.
Her study used different arms to test dosing, drug delivery, and side effects. Of the 58 participants who could be evaluated at the end of the study, about half had their condition stabilize for at least two months.
Participants in the last arm of the study, who had CAR-T cells infused into the spinal fluid as well as the cavity around their brain tumors, had an overall survival of about 10½ months. Typically, people with recurrent glioma tumors could expect to survive about six months after their cancers came back, Brown said.
She said the researchers won’t know whether their treatment extends survival until it is tested in a placebo-controlled trial.
Encouraging results
Researchers at the University of Pennsylvania are taking an approach that combines both the protein targeted used in the City of Hope trials, interleukin-13 receptor alpha 2, and the one targeted by the Mass General study, epidermal growth factor receptor or EGFR.
For their study – published Wednesday in the journal Nature Medicine – the Penn team, led by Dr. Donald O’Rourke, harvested T-cells, engineered them to recognize both of these sites on the tumor and then infused them directly into patients’ brains.
“They’re basically like two little claws,” said O’Rourke, director of the Glioblastoma Multiforme Translational Center of Excellence at the University of Pennsylvania Perlman School of Medicine. “This is a more advanced double targeting approach.”
O’Rourke says that as with Mass General’s results, they saw rapid reductions in brain tumors within days of a single dose of CAR-T cells in the first six participants, and some of those reductions have been sustained for several months.
“Seeing the responses in this difficult patient population has given us a lot of encouragement for this,” O’Rourke said.
Participants in the trial got two different doses of CAR-T cells. Both groups had significant side effects from their treatment, including brain swelling, fevers and headaches.
O’Rourke says researchers are going to have to figure out how to make this safer and easier for patients, maybe by trying to treat some of them earlier in the course of their disease.
“When you treat someone with this powerful treatment in the brain who has a lot of tumor in there, the brain gets very inflamed, and they basically have a long, low-grade kind of chronic weakness syndrome,” he said.
“That is the result, sort of like a low-grade smoldering meningitis, because you’re actually putting immune cells in the spinal fluid, and they’re active, and they’re not supposed to be there,” O’Rourke said. So even though the tumors may disappear, he says patients are not able to be at their best.
He said the people they’ve treated who had smaller tumors at the outset have done better.
Like the other researchers, O’Rourke said it’s too early to know whether these treatments can improve survival, but he feels hopeful.
“Most all of these patients should not be alive now that are still alive,” he said.