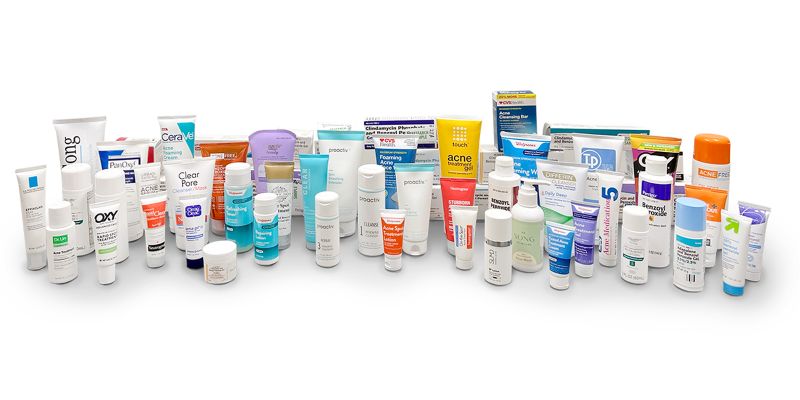
High levels of benzene, a cancer-causing chemical, can form in acne treatment products containing benzoyl peroxide, according to a new report from Valisure, an independent laboratory.
The report says benzene “can form at unacceptably high levels” in both prescription and over-the-counter benzoyl peroxide products, and results from Valisure’s tests showing that some products could form more than 800 times the “conditionally restricted” US Food and Drug Administration concentration limit for benzene.
Tests involving dozens of these products suggest that when benzoyl peroxide acne treatments are stored or handled at high temperatures – such as when left in a hot car at more than 150 degrees Fahrenheit for at least 14 days – they can generate high levels of benzene, the lab announced Wednesday.
In one test, a ProActiv acne product was stored at 158 degrees Fahrenheit for nearly 17 hours. The lab not only detected benzene inside the product, benzene gas was found in the airspace around it – which was the equivalent of air typically found in a compact car – at around 1,270 times the threshold that the US Environmental Protection Agency has for long-term inhalation exposure to benzene, according to the lab.
Other types of acne treatment products that were tested, such as those containing salicyclic acid or adapalene, did not appear to have the issue of forming high levels of benzene, according to the lab.
Benzene is one of the 20 most widely used chemicals in the United States, and people are exposed “mainly by breathing in air containing benzene,” according the American Cancer Society, which was not involved in Valisure’s report.
Benzene is a chemical formed from both natural and human-made processes, and it evaporates into the air very quickly.
It can be used to make chemicals for things like plastics and synthetic fibers, according to the US Centers for Disease Control and Prevention. It’s also used in a variety of products including lubricants, dyes, detergents and drugs.
“Natural sources of benzene include volcanoes and forest fires. Benzene is also a natural part of crude oil, gasoline, and cigarette smoke,” the CDC says.
On Tuesday, Valisure sent a citizen petition to the FDA in which the lab described an initial analysis of 175 acne treatment products, finding that 99 of them contained benzoyl peroxide, and among those products specifically, native benzene was detected in 94.
The petition urges the FDA to “request recalls and a suspension of sales for products containing the active pharmaceutical ingredient benzoyl peroxide.”
An FDA spokesperson said in a statement Wednesday that it has received the citizen petition and will respond directly to the petitioner and post the response in the designated public docket.
“The agency acts on information provided from a variety of sources, such as that provided by Valisure, but such data must be verified as accurate and reproducible before it can be utilized to make regulatory decisions such as recommending product sale suspensions and recalls,” the statement says.
“The agency will continue to provide updates to the public regarding benzene in drug products, as appropriate,” it says. “Drug manufacturers are required to ensure the safety and quality of their drugs.”
Last year, the FDA alerted drug manufacturers about the risk of benzene contamination in certain products such as some hand sanitizers and aerosol drug products, noting that benzene is a known human carcinogen that causes leukemia and other blood disorders.
Regarding benzoyl peroxide products that fall under Reckitt Benckiser’s brand Clearasil, “Reckitt is confident that all Clearasil products, when used and stored as directed on their labels as intended, are safe,” the company said in a statement Wednesday.
“The safety and quality of our products is our top priority and we work closely with regulators around the world to ensure our products are safe and effective for their intended use,” the statement said. “Clearasil products and their ingredients are stable over the storage conditions described on their packaging which represent all reasonable and foreseeable conditions. The findings presented by an independent lab today reflect unrealistic scenarios rather than real-world conditions.”
Valisure and other companies have previously filed patent applications on formulations or techniques to help reduce the rate of benzene formation in products. “No patents have been granted that Valisure is aware of, only applications that were submitted,” Valisure spokesperson Karrah Goldberg said in an email Wednesday.
This isn’t the first time Valisure has sent a citizen petition letter to the FDA regarding concerns about benzene. In 2022, the lab found benzene in dry shampoo products and urged the FDA to request recalls, and in 2021, Valisure detected benzene in sunscreen.
“This discovery of benzoyl peroxide’s fundamental instability and formation of benzene is substantially different than Valisure’s previous findings of benzene in sunscreens, hand sanitizers and other consumer products,” David Light, Valisure’s co-founder and president, said in a statement Wednesday.
“The benzene we found in sunscreens and other consumer products were impurities that came from contaminated ingredients; however, the benzene in benzoyl peroxide products is coming from the benzoyl peroxide itself, sometimes at hundreds of times the conditional FDA limit,” he said. “This means the problem broadly affects benzoyl peroxide products, both prescription and over-the-counter, and necessitates urgent action.”