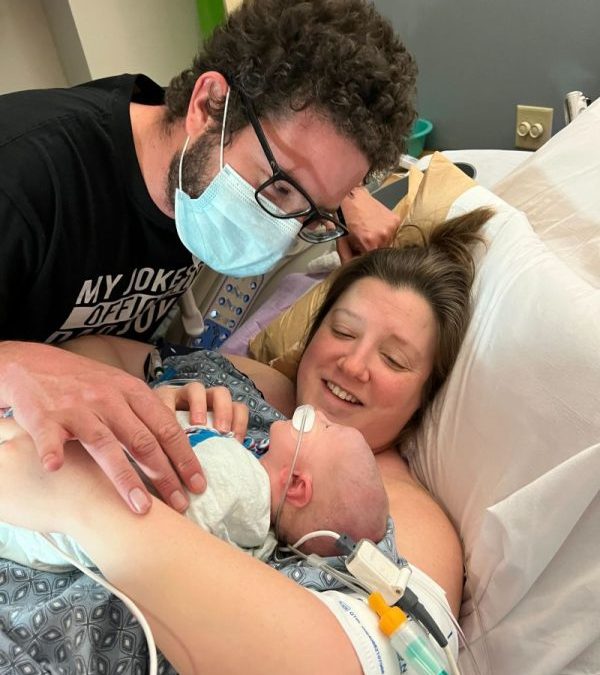
Owen Monroe was 18 days old when he made history, becoming the first person in the world to receive a partial heart transplant.
His groundbreaking surgery, performed in 2022, even captured the attention of Hollywood scriptwriters, who wove his story into a recent episode of the long-running medical drama “Grey’s Anatomy.”
In a study published Tuesday in the journal JAMA, his doctors will document another milestone: For the first time, the tissue used to fix Owen’s heart has grown, a long-sought goal of this type of repair.
At the time of his first operation, Owen’s heart was the size of a strawberry. Today, at 20 months of age, it’s about the size of an apricot – and the new valves and blood vessels have kept up with his growth, which means unlike most children born with the same defect, he may not need to have more risky heart surgeries throughout his life.
Researchers have been working to make growing heart valves a reality through tissue engineering, germinating them from cells in a lab. That approach has worked in animals, but it has not yet panned out in humans.
“This is a huge advance,” said Dr. Kathleen Fenton, chief of the Advanced Technologies and Surgery Branch of the National Heart, Lung, and Blood Institute. She wrote a recent editorial about the potential of partial heart transplants, but she was not involved in this research.
“It’s one child, right? So you have to do research,” Fenton said. “You have to follow these children in the long term and see what really happens. But there is, I think, every reason to hope that it’s really going to be a groundbreaking advance for a subset of children that don’t otherwise have good options.”
The procedure is catching on quickly. Since Owen’s surgery, 12 other partial heart transplants have been performed in children, including nine at Duke Health, the hospital that developed the operation.
The technique has also enabled “domino transplants” and split-root transplants, which allow a single donor heart to save the lives of two critically ill infants.
In a domino transplant, the first child, born with a weak heart muscle that can’t adequately pump blood, gets a whole donated heart while the second baby gets the healthy blood vessels and valves from the first infant. In a split-root transplant, the functioning parts of a heart are donated to two infants.
“What’s a big leap is to be able to potentially make use of donor parts that otherwise couldn’t be used,” Fenton said.
Dr. Joseph Turek, chief of pediatric heart surgery at Duke Health and the surgeon who created the partial heart transplant, said he believes that it could help hundreds of children every year in the US.
“I think that that, ultimately, it will be limited by the number of donors,” said Turek, who is the lead author of the new study.
“There are 500 pediatric heart transplants that we do a year in this country, and so for the vast majority of those kids getting hearts, they would have available their old hearts. We could use their valves. So my guess is, this could help over a thousand kids a year, hopefully,” he said.
A tiny pioneer
Nick and Tayler Monroe, Owen’s parents, learned that he had a serious heart defect when they went for an in-depth ultrasound exam at his 20th week of development.
Owen had a rare birth defect called truncus arteriosis.
Normally, people have two major blood vessels coming out of the tops of their hearts. One, the pulmonary artery, sends blue blood that’s depleted of oxygen to the lungs to pick up more while the other, the aorta, sends oxygen-rich red blood to fuel the rest of the body.
With truncus arteriosis, which affects about 250 babies born in the US each year, these two vessels are fused, allowing oxygenated and deoxygenated blood to mix. They are also lacking a valve needed to keep blood from flowing backward, and many with this condition are born with a hole between the bottom two pumping chambers of their hearts.
Babies with truncus arteriosis are often in distress soon after birth. Too much blood flows into their lungs, straining the tiny air sacs. Because their blood isn’t well-oxygenated, they may look blue and work harder to breathe.
“So even before he was born, we were going in knowing that he was going to have open-heart surgery very young and then was probably going to have a handful of surgeries before he was a teenager and then a couple more after that throughout his life,” Nick Monroe said.
But Owen’s case was even more serious, Turek explained.
Normally, when a child has truncus arteriosis, surgeons need to replace only the pulmonary heart valve they weren’t born with. They’re often able to save and use the single valve the child does have to serve as their aortic valve.
In Owen’s case, even the one valve he had didn’t work well. A doctor who saw his first post-birth ultrasound thought Owen would need a full heart transplant.
But the next day, Turek approached Owen’s parents and presented an alternative: He had been practicing a new technique that could replace just the defective parts of Owen’s heart with living vessels and valves from a recently deceased donor.
Tayler Monroe, Owen’s mom, asked the doctor how many times he had done the procedure.
“He said, ‘I’ve done it five times – on piglets,’ ” Nick Monroe said.
Making the decision to be first
Turek told them that if everything went well, Owen wouldn’t need any more open-heart surgeries. If he repaired Owen’s heart using frozen valves harvested from cadavers, their son had only a 50% chance of survival.
Monroe says that they realized it was risky, but they also knew that their son might not survive any other way. He was already in heart failure. The hospital couldn’t give him a lifesaving heart-lung bypass called ECMO because his damaged heart wouldn’t have been able to handle it.
“So they had no emergency care they could give him, any more than they already were,” Monroe said.
“Everybody always says to us, ‘it must have been such a hard decision,’ but when your backs are against the wall and then your surgeon says, ‘well, here’s a lifeline,’ you take the chance,” he said. “We saw it as the best opportunity for our son to have a chance of survival.”
They agreed to try the partial transplant, and then they waited.
“Every day, he was looking so jaundiced, and you could feel the tension on the floor. Every time we had rounds. How it was weighing on everybody,” Monroe said. “The entire staff of that unit – all the nurses, all the fellows and all the doctors – every time, they’re like, ‘there’s no change. No good news.’ ”
Owen’s mom is a pediatric ICU nurse. She knew all too well what was happening, and Monroe said she coped by separating her emotions from her clinical understanding.
“When we were at the hospital, she was in nurse mode, so she was very analytical and logical and knew what all the numbers did on all the pumps and was really good about asking the doctors questions,” Monroe said.
But when she got back to their room at the Ronald McDonald House at night, Monroe said, he could hear her crying in the shower “because it’s so emotionally draining and you can only separate yourself so far.”
After more than two weeks, they got their first jolt of hope: The hospital had found a matching heart. The donor’s heart muscle wasn’t suitable for transplant, but the valves and blood vessels might help Owen.
Staff relayed the good news the morning of April 22, 2022. By 3 p.m., Owen was in surgery.
“And so we’re just sitting there in the waiting room for the intensive care unit, and hours are going by, and you don’t really want to watch TV or do anything,” Monroe said. “We’re just sort of staring off into space, just waiting for the next phone call.”
Finally, around midnight, Turek came out to tell them the procedure was finished. Everything had gone well.
Since then, there’s been no stopping Owen, who is now a happy, active toddler who is meeting his developmental milestones.
“The most amazing thing about this whole experience is, it’s almost as if his body was ready to go and was like, ‘this is the only thing that’s wrong with me. Just fix my heart, and I’m good to go,’ ” Monroe said.
Weighing the risks
There are tradeoffs with partial heart transplants. Owen still needs medication to suppress his immune system so it won’t reject the transplanted parts of his heart, but he needs only a low dose.
Typically, when the immune system rejects a donor heart as foreign, it is rejecting the heart muscle. There aren’t as many markers in the tissue that makes up blood vessels and valves, so it’s not as reactive.
“Normal heart transplant patients go on two agents to manage their rejection issues. And Owen is on one of those two agents, at basically half a dose,” Turek said.
Turek says they’re still studying how the body responds to this type of transplant to see whether they may be able to refine his regimen even further.
Drugs that suppress immune function can be life-sustaining, but they also make people more vulnerable to infections and cancer. So the goal is to find a long-term fix that won’t require any.
Another child who’s gotten a partial heart transplant hasn’t needed any anti-rejection medication and is doing well.
“He’s on no immunosupression, and his valve continues to grow very well,” Turek said, “I suspect that his donor was very well-matched with him.”
The Monroes are grateful they got to help Owen and ultimately other children, too.
If Owen had gotten the older kind of repair for his heart, Nick Monroe said, they would be planning his third surgery by now.
“When we made the decision, of course, we were only thinking about what was best for our son,” he said. “But at the end of the day, being able to be sort of a pioneer to help other children improve their lives and sort of advance treatment for other sick kids with congenital heart disease … we’re very proud we took the risk.”